
Now Offering Coolsculpting!
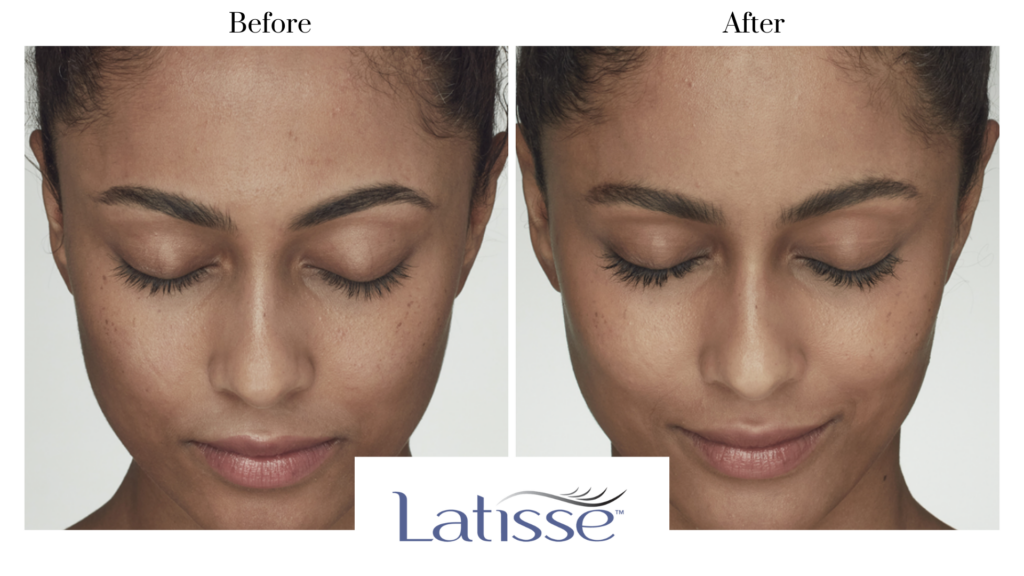
Great eyelashes don’t just happen overnight. Latisse® at Solimar consists of a 16 week, at-home treatment to help give you fuller, longer, and darker eyelashes. The Latisse® solution works gradually and remarkably by first showing changes in length. After changes in length, you will begin to notice an increase in thickness and darkness of your lashes.
Latisse is an FDA-approved treatment to grow eyelashes for people with inadequate or not enough lashes. To learn more about this eyelash solution, contact Solimar Medispa.

Warnings and Precautions: In patients using LUMIGAN® (bimatoprost ophthalmic solution) or other prostaglandin analogs for the treatment of elevated intraocular pressure (IOP), the concomitant use of LATISSE® may interfere with the desired reduction in IOP. Patients using prostaglandin analogs including LUMIGAN® for IOP reduction should only use LATISSE® after consulting with their physician and should be monitored for changes to their intraocular pressure.
Increased iris pigmentation has occurred when bimatoprost solution was administered. Patients should be advised about the potential for increased brown iris pigmentation, which is likely to be permanent.
Bimatoprost has been reported to cause pigment changes (darkening) to periorbital pigmented tissues and eyelashes. The pigmentation is expected to increase as long as bimatoprost is administered, but has been reported to be reversible upon discontinuation of bimatoprost in most patients.
There is the potential for hair growth to occur in areas where LATISSE® solution comes in repeated contact with skin surfaces. Apply LATISSE® only to the skin of the upper eyelid margin at the base of the eyelashes.
LATISSE® solution should be used with caution in patients with active intraocular inflammation (eg, uveitis) because the inflammation may be exacerbated.
Adverse Reactions: The most frequently reported adverse events were eye pruritus, conjunctival hyperemia, skin hyperpigmentation, ocular irritation, dry eye symptoms, and erythema of the eyelid. These events occurred in less than 4% of patients.
Postmarketing Experience: The following reactions have been identified during postmarketing use of LATISSE® in clinical practice: burning sensation (eyelid), erythema periorbital, eye swelling, eyelid irritation, eyelid edema, eyelid pruritus, iris hyperpigmentation, lacrimation increased, madarosis and trichorrhexis (temporary loss of a few eyelashes to loss of sections of eyelashes, and temporary eyelash breakage, respectively), rash (including macular, erythematous, and pruritic limited to the eyelids and periorbital region), skin discoloration (periorbital), and vision blurred.
Use in Specific Populations: Use in pediatric patients below the age of 16 years is not recommended because of potential safety concerns related to increased pigmentation following long-term chronic use.
Warnings and Precautions: In patients using LUMIGAN® (bimatoprost ophthalmic solution) or other prostaglandin analogs for the treatment of elevated intraocular pressure (IOP), the concomitant use of LATISSE® may interfere with the desired reduction in IOP. Patients using prostaglandin analogs including LUMIGAN® for IOP reduction should only use LATISSE® after consulting with their physician and should be monitored for changes to their intraocular pressure.
Our friendly team at Solimar Medispa is here to guide you through your journey to a revitalized and confident self.


Copyright © 2025 Solimar MediSpa | All rights & content reserved